How To Calculate Water Potential
Name:_____________________________________________________
Investigation: Osmosis and Water Potential
 In this lab, yous will observe the process of osmosis and diffusion. You will also acquire how to calculate water potential. If you lot are not familiar with these concepts, brand sure that yous have looked them up in your textbook. If yous don't know what these terms hateful, this lab is not going to brand sense to you.
In this lab, yous will observe the process of osmosis and diffusion. You will also acquire how to calculate water potential. If you lot are not familiar with these concepts, brand sure that yous have looked them up in your textbook. If yous don't know what these terms hateful, this lab is not going to brand sense to you.
OBJECTIVES
- Investigate the processes of osmosis in a model of a membrane system
- Investigate the event of solute concentration on water potential as it relates to living plant tissues
Do 1 - Osmosis Across a Membrane
1. Obtain half-dozen strips of dialysis tubing and tie an knot in one end of each.
2. Cascade approximately xv-20 ml of each of the following solutions into separate numberless.
| Distilled Water | 0.4 M sucrose | 0.viii M sucrose |
| 0.2 M sucrose | 0.vi Thousand sucrose | 1.0 M sucrose |
3. Remove almost of the air from the bag (but leave a little bit of space) and tie the baggie.
4. Blot the bags to remove any saccharide that may accept spilled, check the bags for leaks.
v. Record the mass of each baggie in the data table.
6. Fill six beakers with enough distilled water to encompass your bags. Place a bag in each 1 (keep track of which pocketbook is in which beaker)
vii. Let the purse sit for 20-xxx minutes. -------- While this is running, set upwards potatoes for exercise 3.
Predict what you lot think will happen during the experiment. (Think virtually which bags will lose water and which will gain h2o.)
8. After twenty-30 minutes, remove the baggies from the water, and carefully blot dry and record the terminal weight.
9. To calculate: percent change in mass= (concluding mass-initial mass)/ initial mass. Then multiply answer by 100.
| Contents in Bag | Initial Mass | Concluding Mass | Mass Deviation | Time in Beaker | % Alter in Mass |
| Distilled Water | |||||
| 0.2 Thou | |||||
| 0.iv 1000 | |||||
| 0.6 M | |||||
| 0.eight Yard | |||||
| 1.0 M |
11. Graph the results for your individual data that shows the relationship between %change in mass and the molarity of the solution. The independent variable is on the Ten axis, and the dependent variable is on the Y centrality
Analysis
ane. Draw the relationship between the change in mass and the molarity of the sucrose in the dialysis tube.
Based on scientific principles, did you notice what you expected? If non, propose a reason or possible errors in set-up or data gathering.
2. Why did you calculate the pct modify in mass rather than only using the change in mass?
3. Predict what would happen to the mass of each bag in this experiment if all the numberless were placed in 0.4 M sucrose solution instead of distilled water. Explain your response.
4. A dialysis bag is filled with distilled water and and then placed in a sucrose solution. The bag's initial mass is 20 yard, and its terminal mass is xviii g. Calculate the percent modify of mass, showing your calculations.
Practice two - Determining the Water Potential of Potato Cells
In animal cells, the movement of water into and out of the cell is influenced past the relative concentration of solute on either side of the jail cell membrane. If water moves out of the cell, the cell volition shrink. If water moves into the cell, the cell may swell or fifty-fifty burst. In plant cells, the presence of a prison cell wall prevents the cells from bursting, only pressure level does somewhen build upward inside the jail cell and affects the process of osmosis. When the pressure level inside the cell becomes large plenty, no additional water will accrue in the cell even. So motion of water through the establish tissue cannot be predicted just through knowing the relative solute concentrations on either side of the plant cell wall. Instead, the concept of water potential is used to predict the direction in which h2o will diffuse through living constitute tissues.
In a general sense, the water potential is the trend of water to diffuse from one area to some other. Water potential is expressed in in bars, a metric unit of pressure equal to about 1 atmosphere and measured with a barometer.
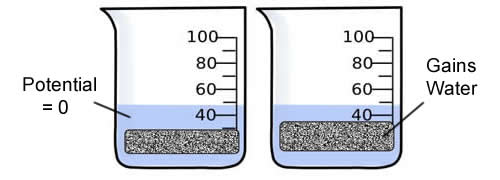
Consider a potato cell is placed in pure water. Initially the water potential outside the prison cell is 0 and is college than the h2o potential inside the cell. Nether these conditions there will be a internet motility of h2o into the cell. The pressure potential within the cell will increment until the prison cell reaches a state of equilibrium.
Directions:
1. Pour 100 mL of your assigned solution (information technology volition be one of the six solutions listed in a higher place in Exercise ii) into a beaker. Slice a potato into 4 equal cylinders or slices, they will resemble french chips.
2. Decide the mass of all 4 tater cylinders together and record.
iii. Identify the cylinders into the beaker with your assigned solution and cover with plastic wrap. Exit overnight.
iv. Remove the cylinders from the beakers and tape the mass. Determine the temperature of the room. ________
5. Consummate the table and graph your results.
| Contents in Bag | Initial Mass | Last Mass | Mass Divergence | %Alter in Mass |
| Distilled H2o | ||||
| 0.ii K | ||||
| 0.4 M | ||||
| 0.six M | ||||
| 0.8 M | ||||
| 1.0 K |
6. Determine the molar concentration of the white potato cores. This would exist the sucrose molarity in which the mass of the potato core does not alter. To notice this, draw the directly line on your graph that best fits your information. The point at which this line crosses the 10 axis represents the molar concentration of sucrose with a h2o potential that is equal to the potato tissue water potential.
What is the Molar concentration of the cores? ___________
seven. Calculate the solute potential ( Ψ ) for the sucrose solution using the formula beneath. Show your piece of work!
Solute Potential Formula: Ψ = -iCRT
i = ionization constant (for sucrose, this is 1 because sucrose does not ionize in water)
C = molar sucrose concentration at equilibrium (determined from graph)
R = pressure constant (0.0831 liter bar/mole °K ) | T = temperature °Thou (273 + °C )
viii. Explain water potential and depict how it affects osmosis.
9. Explain how you would determine the molarity of a potato.
Extension: Design an Experiment to Test an Unknown
You are given a solution of sucrose that has an unknown molarity (.2, .4,.six, .8, i.0), how could you use potatoes, distilled water, or other known solutions to decide the molarity of your unknown? - Be clear in your blueprint, use another page and staple to this i. Conduct your experiment and include the results with your decision of which solution you had. Solutions volition be color-coded by the instructor. Complete the CER chart beneath.
Claim:
Testify:
Reasoning:
Advise means to improve your experimental pattern or to obtain greater conviction in your claim. If you were given another day to work on this, what would you practise?
How To Calculate Water Potential,
Source: https://www.biologycorner.com/worksheets/osmosis-water-potential.html
Posted by: gearhartruss1964.blogspot.com


0 Response to "How To Calculate Water Potential"
Post a Comment